1848
Lord Kelvin (William Thomson), wrote in his paper, On an Absolute Thermometric Scale, of the need for a scale whereby "infinite cold" (absolute zero) was the scale’s null point, and which used the degree Celsius for its unit increment. Thomson calculated that absolute zero was equivalent to −273 °C on the air thermometers of the time.[1] This absolute scale is known today as the Kelvin thermodynamic temperature scale. Thomson’s value of "−273" was the reciprocal of 0.00366 - the accepted expansion coefficient of gas per degree Celsius relative to the ice point, giving a remarkable consistency to the currently accepted value
Kelvin
The kelvin (symbol: K) is a unit increment of temperature and is one of the seven SI base units. The Kelvin scale is a thermodynamic (absolute) temperature scale referenced to absolute zero, the theoretical absence of all thermal energy. By definition it is zero kelvin (0 K). The secondary reference point on the Kelvin scale is the triple point of water (0.01 degrees Celsius). The Kelvin scale is the difference between these two reference points, with the kelvin defined as one 273.16th of this scale. The Kelvin scale and the kelvin are named after the Belfast-born physicist and engineer William Thomson, 1st Baron Kelvin (1824–1907), who wrote of the need for an "absolute thermometric scale". Unlike the degree Fahrenheit and degree Celsius, the kelvin is not referred to as a "degree", nor is it typeset with a degree symbol; that is, it is written K and not °K. The kelvin and the degree Celsius are often used together, as they have the same interval, and 0 kelvin is −273.15 degrees Celsius
Lord Kelvin (William Thomson), wrote in his paper, On an Absolute Thermometric Scale, of the need for a scale whereby "infinite cold" (absolute zero) was the scale’s null point, and which used the degree Celsius for its unit increment. Thomson calculated that absolute zero was equivalent to −273 °C on the air thermometers of the time.[1] This absolute scale is known today as the Kelvin thermodynamic temperature scale. Thomson’s value of "−273" was the reciprocal of 0.00366 - the accepted expansion coefficient of gas per degree Celsius relative to the ice point, giving a remarkable consistency to the currently accepted value
Kelvin
The kelvin (symbol: K) is a unit increment of temperature and is one of the seven SI base units. The Kelvin scale is a thermodynamic (absolute) temperature scale referenced to absolute zero, the theoretical absence of all thermal energy. By definition it is zero kelvin (0 K). The secondary reference point on the Kelvin scale is the triple point of water (0.01 degrees Celsius). The Kelvin scale is the difference between these two reference points, with the kelvin defined as one 273.16th of this scale. The Kelvin scale and the kelvin are named after the Belfast-born physicist and engineer William Thomson, 1st Baron Kelvin (1824–1907), who wrote of the need for an "absolute thermometric scale". Unlike the degree Fahrenheit and degree Celsius, the kelvin is not referred to as a "degree", nor is it typeset with a degree symbol; that is, it is written K and not °K. The kelvin and the degree Celsius are often used together, as they have the same interval, and 0 kelvin is −273.15 degrees Celsius
Celsius
Celsius (known until 1948 as centigrade) is a temperature scale that is named after the Swedish astronomer Anders Celsius (1701–1744), who developed a similar temperature scale two years before his death. The degree Celsius (°C) can refer to a specific temperature on the Celsius scale as well as a unit to indicate a temperature interval (a difference between two temperatures or an uncertainty).
From 1744 until 1954, 0 °C was defined as the freezing point of water and 100 °C was defined as the boiling point of water, both at a pressure of one standard atmosphere.[citation needed] Although these defining correlations are commonly taught in schools today, by international agreement the unit "degree Celsius" and the Celsius scale are currently defined by two different points: absolute zero, and the triple point of VSMOW (specially prepared water). This definition also precisely relates the Celsius scale to the Kelvin scale, which defines the SI base unit of thermodynamic temperature (symbol: K). Absolute zero, the hypothetical but unattainable temperature at which matter exhibits zero entropy, is defined as being precisely 0 K and −273.15 °C. The temperature value of the triple point of water is defined as being precisely 273.16 K and 0.01 °C.[1]
This definition fixes the magnitude of both the degree Celsius and the kelvin as precisely 1 part in 273.16 parts, the difference between absolute zero and the triple point of water. Thus, it sets the magnitude of one degree Celsius and that of one kelvin as exactly the same. Additionally, it establishes the difference between the two scales' null points as being precisely 273.15 degrees Celsius (−273.15 °C = 0 K and 0 °C = 273.15 K).[2]
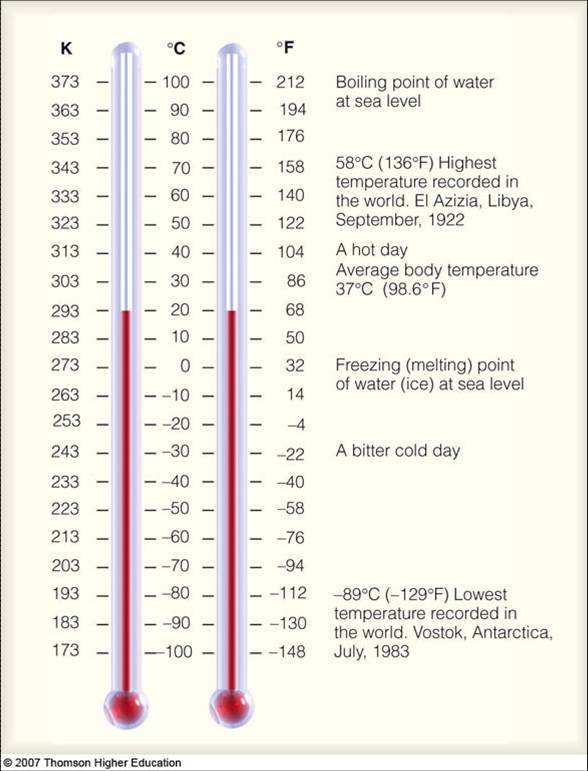
Coverting Celsius To Kelvin
Degrees C +273 = degrees in Kelvin
30 C + 273 = 303 K
0 K - 273 = -273 C (absolute Zero0
No comments:
Post a Comment